![]()
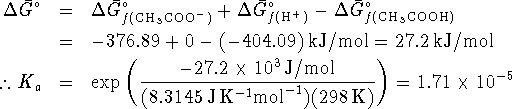
However
![]()
The activity of acetate is equal to that of the protons because
the two are produced in equal quantities by dissociation.
Furthermore, since ![]() is quite small, we can take
is quite small, we can take ![]() . Solving the equilibrium condition for
. Solving the equilibrium condition for
![]() , we get
, we get
![]()
(Note that the small size of this number confirms the validity of the approximation made with respect to the degree of dissociation.)
![]()
![]()
The maximum PG synthesis will occur when ![]() (no free energy is being wasted), i.e. when
(no free energy is being wasted), i.e. when
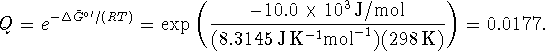
But
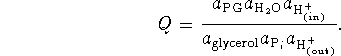
We use the usual approximation that
![]() . Then
. Then
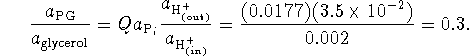
![]()
![]() is just the inverse of the density:
is just the inverse of the density:
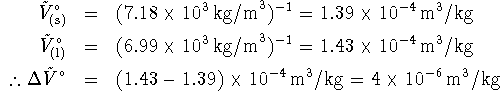
To get ![]() , we note that at the normal
melting point, solid and liquid are in equilibrium. Therefore
, we note that at the normal
melting point, solid and liquid are in equilibrium. Therefore
![]() which implies that
which implies that
![]()
Thus we have
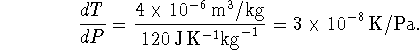
We know the melting point at 1atm and we want to know it at 0atm so consider a change in pressure of -1atm (-101325Pa):
![]()
The melting point of tin in a vacuum is therefore virtually identical
to its melting point at 1atm, i.e. we may expect tin to melt
very near ![]() in a vacuum.
in a vacuum.
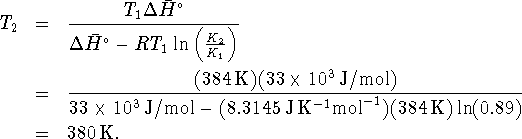
In degrees Celcius, this is ![]() .
.
![]()
is negative. Thus, ![]() (at the higher temperature
(at the higher temperature ![]() ) is
lower than
) is
lower than ![]() which is precisely as predicted by Le
Chatelier's principle. Conversely, for an endothermic reaction,
the right-hand side of the equation is positive implying that
which is precisely as predicted by Le
Chatelier's principle. Conversely, for an endothermic reaction,
the right-hand side of the equation is positive implying that
![]() which agrees with Le Chatelier's principle since in
this case heat is absorbed by forming more products.
which agrees with Le Chatelier's principle since in
this case heat is absorbed by forming more products.