Chemistry 2720, Fall 96, Assignment 1
Due: September 24
All questions are equally weighted. Most questions will require data
not provided here. Sources for thermodynamics data include your
textbook (esp. the Appendix starting on p 746 and the table of
properties of water on p 33) and the course web site
(http://people.uleth.ca/~roussel/C2720).
- Calculate the work done when a gas expands from a volume of
1L to 10L against a constant external pressure of 1atm.
(Hint and caution: The answer should be in SI units.)
- An electric kettle has a 1500W heating element. It is filled
with 1.5L of water at
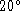 C.
Assume that the kettle itself has a negligible heat capacity and
that no heat is lost from the water during the heating process.
How long does it take the kettle to bring the water
to a boil?
C.
Assume that the kettle itself has a negligible heat capacity and
that no heat is lost from the water during the heating process.
How long does it take the kettle to bring the water
to a boil? - Lead melts at
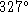 C with a latent heat of fusion of 25J/g.
The specific heat capacity of lead is 0.127JK
C with a latent heat of fusion of 25J/g.
The specific heat capacity of lead is 0.127JK 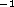 g
g 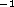 .
Small spheres of uniform size
are frequently made by dropping molten metal into water. A
surface tension effect causes
the metal to form almost perfect spheres and the water
cools the metal very rapidly, causing it to solidify. Suppose that
lead spheres each weighing 0.5g are being made by this process in a
1kg bucket of water. The spheres are removed quickly and
their mean temperature on removal is found to be
.
Small spheres of uniform size
are frequently made by dropping molten metal into water. A
surface tension effect causes
the metal to form almost perfect spheres and the water
cools the metal very rapidly, causing it to solidify. Suppose that
lead spheres each weighing 0.5g are being made by this process in a
1kg bucket of water. The spheres are removed quickly and
their mean temperature on removal is found to be 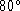 C.
Assume that little heat is lost from the water to its environment
and that negligible
evaporation takes place. For safety reasons, it is desired to
keep the water temperature below
C.
Assume that little heat is lost from the water to its environment
and that negligible
evaporation takes place. For safety reasons, it is desired to
keep the water temperature below 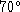 C at all times. If
the water
starts out at
C at all times. If
the water
starts out at 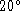 C, how many individual
lead spheres can be made
before the water
must be changed?
C, how many individual
lead spheres can be made
before the water
must be changed? - In mammals, 1g of carbohydrates produces a maximum of 4.0kcal
of useful
work. A calorie is 4.184J.
A shipment consisting of 24 boxes each weighing 10kg
arrives at your office
which you must lift to a shelf 1.5m off the ground. From your
elementary physics, you recall that the work done on a
mass m in raising it to a height h is mgh, where
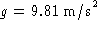 is the acceleration due to gravity.
Instead of doing the job right away, you decide to procrastinate
by solving a thermodynamics problem first:
How much spaghetti (almost a pure carbohydrate)
should you eat after carrying out this task
to compensate for the work you will have done and avoid losing weight?
is the acceleration due to gravity.
Instead of doing the job right away, you decide to procrastinate
by solving a thermodynamics problem first:
How much spaghetti (almost a pure carbohydrate)
should you eat after carrying out this task
to compensate for the work you will have done and avoid losing weight? -
- Compute the standard enthalpy of combustion of acetylene
to carbon dioxide and liquid water.
- Compute the standard change in internal energy during the
combustion of acetylene. Treat all gases as ideal.
Marc Roussel
Thu Sep 12 09:13:33 MDT 1996
![]()