Hint: The derivation is mostly like that of ordinary particles
in boxes, except that you can't use
![]() since
photons have no mass.
since
photons have no mass.
- (a)
- The fundamental vibration frequency of
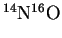 is
is
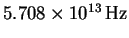 .
Calculate the force constant for
this molecule.
.
Calculate the force constant for
this molecule.
- (b)
- Force constants depend only on electronic
effects and so are identical for isotopic variants.
Estimate the fundamental vibration frequency of
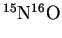 .
.
- (a)
- Chlorophyll a absorbs photons across a range of wavelengths. The absorption maximum is at 680nm. The energy is used to fix carbon, i.e. to form carbohydrates from carbon dioxide and water. It takes about 485kJ to fix one mole of carbon. What is the minimum number of moles of 680nm photons required to fix one mole of carbon?
- (b)
- Experimentally, it is found that it takes 8 to 9 photons per carbon atom fixed. Quantum yield, a measure of efficiency for photochemical processes, is defined as the number of photons required divided by the actual number of photons involved in the process. What is the quantum yield for photosynthesis?
- (a)
- The absorption rotational spectrum of the linear molecule
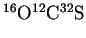 shows absorption lines at 24.32592, 36.48882, 48.65164 and
60.81408GHz. Calculate the moment of inertia of this
molecule.
shows absorption lines at 24.32592, 36.48882, 48.65164 and
60.81408GHz. Calculate the moment of inertia of this
molecule.
- (b)
- To which transition does each of the above lines correspond?
- (c)
- Predict the rotational Raman spectrum of this molecule if the exciting radiation has a wavelength of 336.732nm.