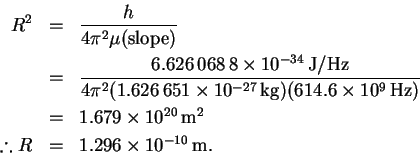Up: Back to the Chemistry 2720 assignments index
Chemistry 2720 Fall 2000 Assignment 8 Solutions
- 1.
- Rn
- 7s25f146d107p2
- (a)
- There should be a
Uuq2+ ion obtained by
removing the p electrons with a ground-state
configuration of [Rn]7s25f146d10.
There should also be a
Uuq4+ ion whose
ground state is [Rn]5f146d10.
- (b)
- The atom should be paramagnetic since its p electrons
are unpaired. On the other hand, both of the ions have
only paired electrons, so they should be diamagnetic.
- 2.
- (a)
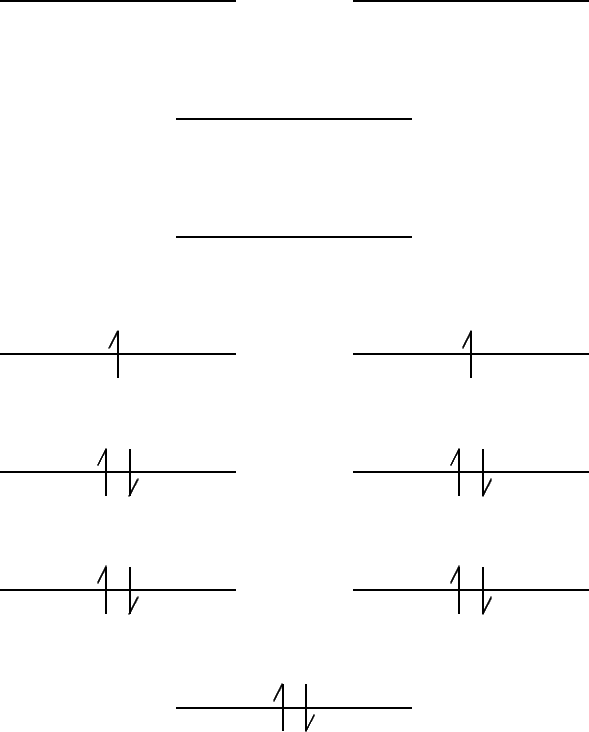
- The chromium atom has six valence electrons, so the
Cr2 molecule has twelve valence electrons.
The orbitals are filled as follows:
- (b)
- Since there are unpaired electrons,
Cr2 should
be paramagnetic.
- (c)
- i.
- It is a bonding orbital with an enhanced electron
density between the two nuclei.
- ii.
- This orbital looks like it results from adding
3d0 atomic orbitals on each chromium
atom.
- 3.
- (a)
- Raman only
- (b)
- both absorption and Raman
- (c)
- both absorption and Raman
- (d)
- neither
- 4.
- Straightforward method:
- The lines of the rotational
spectrum of a diatomic molecule are spaced by
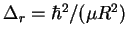 .
The spacings (obtained by subtracting the frequencies of
adjacent lines)
are as follows: 624, 617, 617, 619, 615, 610, 611 and 607GHz.
The average is
.
The spacings (obtained by subtracting the frequencies of
adjacent lines)
are as follows: 624, 617, 617, 619, 615, 610, 611 and 607GHz.
The average is
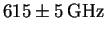 .1
We need to convert this quantity to energy units:
.1
We need to convert this quantity to energy units:
We also need the reduced mass:
To convert amu to kg, since an amu is the same as a
g/mol, we divide by Avogadro's number and by 1000. The
result is
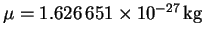 .
We can now calculate R:
.
We can now calculate R:
- By fitting:
- We don't know the transition (i.e. the value of J)
to which the first line reported corresponds. However,
we do know that adjacent lines differ by one unit of
J. Suppose that the first line corresponds to J=J0.
Then the second line is J0+1, the next J0+2, and
so on. We can therefore rewrite the equation for the
transition energies in the form
where
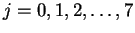 .
If we divide this equation by
h, we get
.
If we divide this equation by
h, we get
If we plot the frequencies vs our (arbitrary) numbering
(j), we should get a straight line of slope
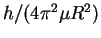 :
The slope of this line is
:
The slope of this line is
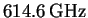 .
Thus
.
Thus
Footnotes
- ...
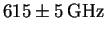 .1
.1
- Note
that it was not necessary for you to carry out an error
analysis. I am performing such an analysis to show you
the accuracy of this method for determining bond
lengths.



Up: Back to the Chemistry 2720 assignments index
Marc Roussel
2000-12-05
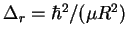 .
The spacings (obtained by subtracting the frequencies of
adjacent lines)
are as follows: 624, 617, 617, 619, 615, 610, 611 and 607GHz.
The average is
.
The spacings (obtained by subtracting the frequencies of
adjacent lines)
are as follows: 624, 617, 617, 619, 615, 610, 611 and 607GHz.
The average is
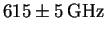 .1
We need to convert this quantity to energy units:
.1
We need to convert this quantity to energy units:
![\begin{eqnarray*}\Delta_r & = & h\Delta\nu_r\\
& = & (6.626\,068\,8\times
10^...
...athrm{Hz}]\\
& = & (4.08\pm 0.03)\times 10^{-22}\,\mathrm{J}.
\end{eqnarray*}](img4.gif)
![\begin{eqnarray*}\mu & = & (m_\mathrm{H}^{-1}+m_\mathrm{Cl}^{-1})^{-1}\\
& = &...
...,amu)^{-1}\right]^{-1}\\
& = & 0.979\,592\,539\,\mathrm{amu}.
\end{eqnarray*}](img5.gif)
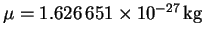 .
We can now calculate R:
.
We can now calculate R:
![\begin{eqnarray*}R & = & \frac{\hbar}{\sqrt{\mu\Delta_r}}\\
& = & \frac{h}{2\p...
...rm{J}]}}\\
& = & (1.295\pm 0.005)\times 10^{-10}\,\mathrm{m}.
\end{eqnarray*}](img7.gif)
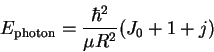
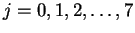 .
If we divide this equation by
h, we get
.
If we divide this equation by
h, we get
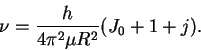
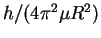 :
The slope of this line is
:
The slope of this line is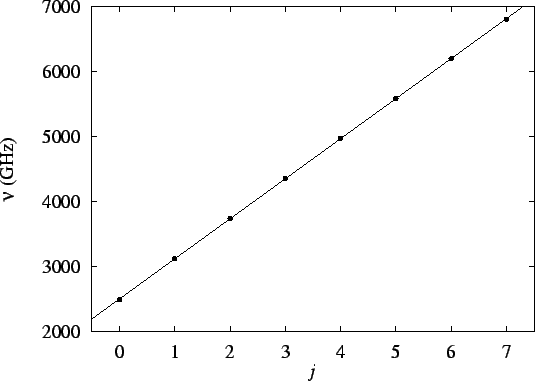
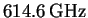 .
Thus
.
Thus