


Up: Back to the Chemistry 2720 assignments index
Chemistry 2720 Fall 2000 Assignment 7
Due: Tuesday, Nov. 14, 9:25a.m.
- 1.
- (a)
- Derive an equation for the energy levels of a particle confined
to a ring of radius r. [10 marks]
- (b)
- This might be a (very)
crude model for the
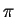 electron system of
benzene. Benzene has six
electron system of
benzene. Benzene has six 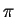 electrons confined to a
hexagon of radius 1.4Å. Because of the Pauli
exclusion principle, each orbital (energy level) can
hold two electrons so the lowest three energy levels are
filled. Calculate the wavelength of the transition from
the highest occupied orbital to the lowest unoccupied
orbital. [6 marks]
electrons confined to a
hexagon of radius 1.4Å. Because of the Pauli
exclusion principle, each orbital (energy level) can
hold two electrons so the lowest three energy levels are
filled. Calculate the wavelength of the transition from
the highest occupied orbital to the lowest unoccupied
orbital. [6 marks]
- 2.
- The atomic emission spectrum of a light atom can contain
lines corresponding to its hydrogenic ion.
Give the wavelengths and colors of a few of the
visible
lines of
Li2+. Confine your attention to lines
involving transitions between levels with principal quantum numbers
of 10 or less.
[10 marks]
- 3.
- The emission spectrum of hydrogen has a line at 410.2nm.
To what transition does this line correspond?
[8 marks]
Marc Roussel
2000-11-04
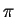 electron system of
benzene. Benzene has six
electron system of
benzene. Benzene has six 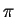 electrons confined to a
hexagon of radius 1.4Å. Because of the Pauli
exclusion principle, each orbital (energy level) can
hold two electrons so the lowest three energy levels are
filled. Calculate the wavelength of the transition from
the highest occupied orbital to the lowest unoccupied
orbital. [6 marks]
electrons confined to a
hexagon of radius 1.4Å. Because of the Pauli
exclusion principle, each orbital (energy level) can
hold two electrons so the lowest three energy levels are
filled. Calculate the wavelength of the transition from
the highest occupied orbital to the lowest unoccupied
orbital. [6 marks]