Note: Methanol boils at
![]() .
.
- (a)
- In a fuel cell, a fuel is fully oxidized in an isothermal
reaction which occurs in aqueous solution. The oxygen is
taken from the air (partial pressure of oxygen 0.2atm)
so that only the fuel itself need be carried. What is
the maximum electrical work which can be obtained per
kilogram of the fuel hydrogen in a fuel cell operating
at
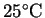 if a pressure regulator supplies
the hydrogen at a pressure of 1atm and pure water
is produced? [10 marks]
if a pressure regulator supplies
the hydrogen at a pressure of 1atm and pure water
is produced? [10 marks]
- (b)
- How much electrical work is produced per kilogram of
hydrogen at
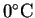 ? At
? At
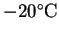 ? Fuel cells are currently being
developed for use as power sources in automobiles.
Would you advise using an
electrical heater to warm fuel cells installed in
cars to be sold in Canada? [10 marks]
? Fuel cells are currently being
developed for use as power sources in automobiles.
Would you advise using an
electrical heater to warm fuel cells installed in
cars to be sold in Canada? [10 marks]
is used to compute the Gibbs free energy of reaction under particular conditions. The enthalpy change depends extremely weakly on the reaction conditions. Use this fact to derive an equation relating the entropy change to the standard entropy change and to