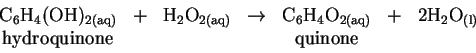
is highly exothermic and actually causes the solvent (water) to boil.
- (a)
- Given
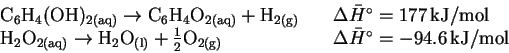
and the table of enthalpies of formation in the textbook, calculate the standard enthalpy change for the reaction of hydroquinone with hydrogen peroxide. [5 marks] - (b)
- Insects are poikilothermic, i.e. they don't generate
heat internally and their body
temperature is thus roughly the same as the ambient temperature
at any given time.
Assuming that the enthalpy change is roughly constant
and that the presence of solutes has
little effect on the properties of
water,1
what are the minimum
concentrations of hydroquinone and of hydrogen peroxide
required to bring the solvent to a boil from an ambient
temperature of
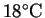 ?
[10 marks]
Hint: How many moles of the reactants does it take to
bring one liter of water to a boil?
?
[10 marks]
Hint: How many moles of the reactants does it take to
bring one liter of water to a boil?
- (c)
- Calculate the standard change in internal energy for the decomposition of hydroquinone to hydrogen and quinone. [5 marks]