
Up: Back to the Chemistry 2710 test
index
Chemistry 2710 Spring 2000 Test 1
Name:
Aids allowed: calculator, one  sheet of notes
sheet of notes
Please write your answers in ink. If you have a graphing calculator,
you can use it rather than hand drawing graphs. If you do use your
calculator's graphing capabilities, explain in
detail what you did (what graphs you drew, how you interpreted them,
etc.). If you need to draw graphs by hand, graph paper is included at
the end of this exam. Make sure to label your graphs with
the question number.
Advice: Don't waste time. There should be plenty of time to answer
all questions, provided you start by picking up the easy marks. Don't
force yourself to work on the questions in the order
in which they appear on this paper.
- For the reaction of ozone with nitrogen monoxide
(
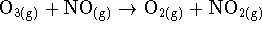 ) at 340K, the following initial rate data have been obtained:
) at 340K, the following initial rate data have been obtained:
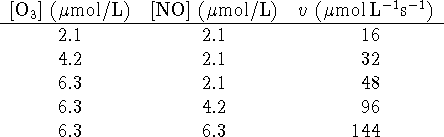
- Determine the empirical rate law and the value of the rate
constant. [9 marks]
- Could this reaction possibly be elementary?
Explain briefly. [2 marks]
- Consider the mechanism

- What is the overall reaction? [4 marks]
- If
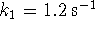 ,
,
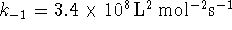 ,
, 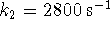 and
and 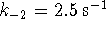 ,
what is the
equilibrium constant for the overall reaction?
[9 marks]
,
what is the
equilibrium constant for the overall reaction?
[9 marks]
- For the essentially irreversible reaction
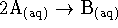 ,
the following data were obtained:
,
the following data were obtained:
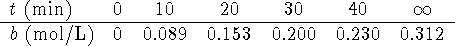
- Previous experiments showed that the rate did not depend on b.
The order of the reaction with respect to A is either
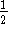 or 1. Can these data be used to
determine the order? If so, report the order and rate
constant. If not, demonstrate that the data are
inadequate to this purpose. [9 marks]
or 1. Can these data be used to
determine the order? If so, report the order and rate
constant. If not, demonstrate that the data are
inadequate to this purpose. [9 marks]
Hint: The amount of B which accumulates after a long
time can be used to calculate the initial amount of A.
- Could this reaction possibly be elementary?
Explain briefly. [2 marks]
- Cell division is triggered (in part) by the accumulation of a
protein called cyclin in a cell. Although the mechanism by
which cyclin interacts with the cell-cycle machinery is complex, we
can study a simplified version of this system by only
considering cyclin accumulation and degradation.
- Suppose that the mechanism is

(zero-order production, first-order degradation)
where C represents cyclin.
Write the rate law for the concentration of cyclin from
this mechanism. [2 marks]
- Verify that

is a solution to your rate equation if c(0)=0.
[8 marks]
- Suppose that the
rate constants appearing in the above mechanism have
the values
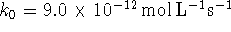 and
and 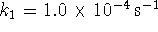 . Moreover, suppose that cyclin must
accumulate to a level of 15nmol/L to trigger division.
How long does this take? [5 marks]
. Moreover, suppose that cyclin must
accumulate to a level of 15nmol/L to trigger division.
How long does this take? [5 marks]

Up: Back to the Chemistry 2710 test
index
Marc Roussel
Fri Feb 18 09:59:48 MST 2000
![]() sheet of notes
sheet of notes
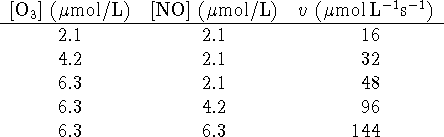

![]()
![]()
![]()