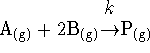 has a rate law
has a rate law
Name:
Suppose that the reaction
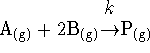 has a rate law
has a rate law
![]() with
with ![]() at
320K.
The reaction is carried out in a glass bulb of fixed volume at 320K.
If the initial pressures of A and B are, respectively, 40 and
30kPa, how long
would it take to completely use up the limiting reagent?
What is the total pressure at the end of the reaction?
at
320K.
The reaction is carried out in a glass bulb of fixed volume at 320K.
If the initial pressures of A and B are, respectively, 40 and
30kPa, how long
would it take to completely use up the limiting reagent?
What is the total pressure at the end of the reaction?
![]() and
and ![]() .
.
For a reaction of general order, ![]() .
.