![]()
As can be seen from the following graph, the data fit a line very well:
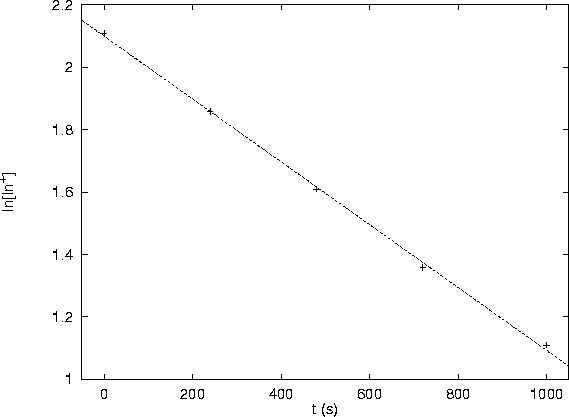
This confirms the rate law.
![]()
where x represents the concentration of indium (I) ions. Accordingly,
![]()
If this much ![]() remains at 900s, then
remains at 900s, then
![]() has reacted. Since the
volume is 0.4L, the number of moles of indium (I) ions which
have reacted is 1.96mmol. By stoichiometry, the number of
moles of indium metal formed is therefore
has reacted. Since the
volume is 0.4L, the number of moles of indium (I) ions which
have reacted is 1.96mmol. By stoichiometry, the number of
moles of indium metal formed is therefore ![]() . The mass of indium metal formed is
therefore
. The mass of indium metal formed is
therefore ![]() or 0.15g.
or 0.15g.