![]()
![]()
From the first two points, we calculate
![]()
From the next two points, we get
![]()
Since (within the accuracy of the data) we get the same rate
constant in both cases, we conclude that this is a first-order
reaction with ![]() .
.
- We use the equation
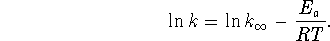
The first experimental point tells us that
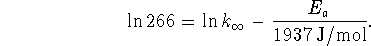
From the second point, we have
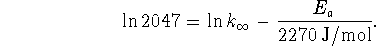
Subtract the two equations to eliminate
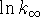 :
:
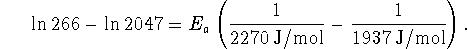
Therefore
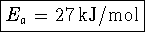
- Bonus:
- Substitute
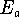 into one of your two original
equations and solve for
into one of your two original
equations and solve for 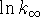 :
:
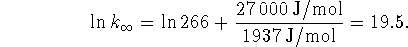
Use
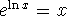 to get the
to get the 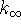 out of the
exponent. Recall that
out of the
exponent. Recall that 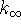 has the same units as
k:
has the same units as
k:
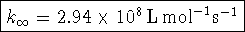
- The equations are obtained by applying the law of mass
action:
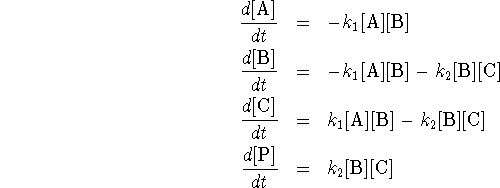
-
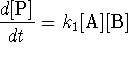
- Bonus:
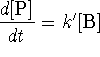 where
where 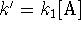 is
approximately constant.
is
approximately constant.
![]()
From the first-order equation, we have
![]()
which is
![]()