![]()
The rate constant can be obtained from (say) the first line of the table using the rate law:
![]()
![]()
k is computed from the half-life:
![]()
![]()
(I believe that the true detection limits are somewhat lower so that the carbon dating method can be used to determine dates of samples somewhat older than this calculation shows.)
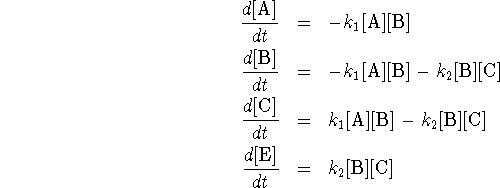
A and B are reactants, C is an intermediate and P is a product.
![]()
The second data point gives us
![]()
Subtract the two equations to eliminate ![]() :
:
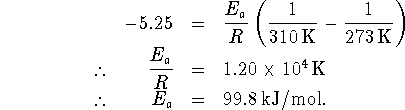
Now substitute this data back into one of the two original equations, say the first one:
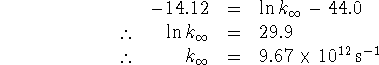
- The half-life is the time required for half the original quantity of a reactant to be removed.
- At
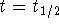 ,
, 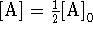 .
Substituting these values into the third-order equation,
we have
.
Substituting these values into the third-order equation,
we have
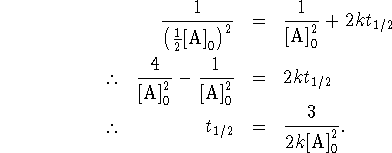
- The rate constant for a third-order process is in
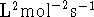 .
. - If we plot
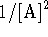 as a function of time, we get
a straight line of slope 2k. The rate constant is therefore
extracted by dividing the slope of the plot by 2.
as a function of time, we get
a straight line of slope 2k. The rate constant is therefore
extracted by dividing the slope of the plot by 2.