![]()
is ![]() at 298K. If the standard free energy of
formation of liquid water is -237.2kJ/mol, what is the
standard free
energy of formation of the hydroxide ion?
at 298K. If the standard free energy of
formation of liquid water is -237.2kJ/mol, what is the
standard free
energy of formation of the hydroxide ion?
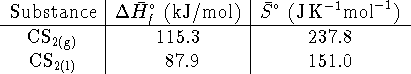
Hint: What is ![]() at the boiling point?
at the boiling point?
![]()

![]()
is ![]() at 298K. If the standard free energy of
formation of liquid water is -237.2kJ/mol, what is the
standard free
energy of formation of the hydroxide ion?
at 298K. If the standard free energy of
formation of liquid water is -237.2kJ/mol, what is the
standard free
energy of formation of the hydroxide ion?
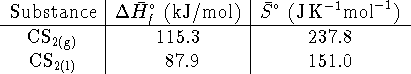
Hint: What is ![]() at the boiling point?
at the boiling point?
![]()
