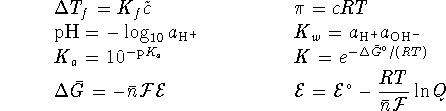Up: Back to the Chemistry 2000 test
index
Chemistry 2000, Fall 1996 Final examination
You have three hours to complete this exam. You may not leave the
examination hall in the first hour or in the last 10 minutes.
The aggregate value of all questions on this exam is 100.
Your answers must be recorded in the exam booklets provided. Please
note the useful information at the end of the paper.
- If a chemical compound which is destroyed by a
first-order reaction has a half-life of 18s, how long
would it take before less than 0.1% of the original quantity
remains? [5 marks]
- The rate of formation of a product P in a chemical reaction is
measured as a function of the concentration of a reactant R
and the following
results are obtained:
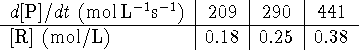
What is the rate law for this reaction? [4 marks]
- What is the pH of a solution made by dissolving 100g of
potassium hydroxide in 500mL of water at 298K? The molar mass of KOH
is 56.1056g/mol. [8 marks]
- Would the solubility of calcium hydroxide be higher in a pH 4
buffer or in a pH 10 buffer? Explain briefly. [3 marks]
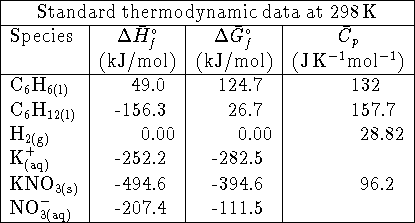
- The enthalpy of formation of liquid boron trichloride is
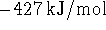 at 298K. The enthalpy of vaporization
of boron trichloride at this temperature is 23kJ/mol. What is the
enthalpy of formation of gaseous
at 298K. The enthalpy of vaporization
of boron trichloride at this temperature is 23kJ/mol. What is the
enthalpy of formation of gaseous 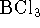 ? Hint: Start
by writing down the
reactions to which the various enthalpies refer. [4 marks]
? Hint: Start
by writing down the
reactions to which the various enthalpies refer. [4 marks] - Calculate the equilibrium constant for the reaction
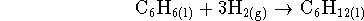
at 298K. [4 marks]
- Ozone (
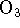 ) reacts with molecular bromine in basic solution.
The products are molecular oxygen and bromate ions (
) reacts with molecular bromine in basic solution.
The products are molecular oxygen and bromate ions ( 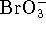 ).
Balance the reaction. [6 marks]
).
Balance the reaction. [6 marks] - A sugar is isolated from a natural source. A solution of the
purified sugar is prepared by dissolving 10.00g of it in water
in a
100.00mL volumetric flask. The osmotic pressure of the
solution at
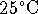 is 719kPa. What is the molar
mass of the sugar? [10 marks]
is 719kPa. What is the molar
mass of the sugar? [10 marks] - 3.5g of potassium nitrate (molar mass 101.1032g/mol)
is dissolved in 100g of water
initially at
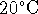 .
Calculate the final
temperature of the solution assuming that no heat is lost to the
surroundings.
The specific heat capacity of
water is
.
Calculate the final
temperature of the solution assuming that no heat is lost to the
surroundings.
The specific heat capacity of
water is 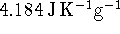 . Aqueous ions have a
negligible heat capacity. [10 marks]
. Aqueous ions have a
negligible heat capacity. [10 marks] - 15g of sodium dihydrogen phosphate (
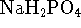 , molar
mass 119.98g/mol)
is dissolved in 150mL of water. What is the pH of the
solution? The
, molar
mass 119.98g/mol)
is dissolved in 150mL of water. What is the pH of the
solution? The 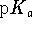 of the first proton of the dihydrogen phosphate anion
is 7.2 at 298K. [10 marks]
of the first proton of the dihydrogen phosphate anion
is 7.2 at 298K. [10 marks] - The solubility product of silver sulfate (
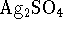 ) is
) is
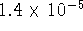 at 298K. What is the solubility of this
compound, expressed in g/L? The molar mass of silver sulfate is
203.97g/mol. [10 marks]
at 298K. What is the solubility of this
compound, expressed in g/L? The molar mass of silver sulfate is
203.97g/mol. [10 marks] - Suppose that you want to generate an electrical current from the
following half-reactions:
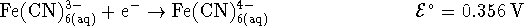

The standard reduction potentials are given for a temperature of
298K.
- Describe the apparatus you would need to obtain
electricity from these reactions. A carefully labeled
sketch is adequate. [6 marks]
- Suppose that a coworker has prepared
the following solutions:
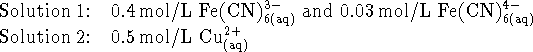
What is the spontaneous overall reaction which occurs in the
apparatus you described above and what voltage is
produced at 298K?
[10 marks]
- For the reaction
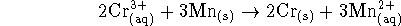
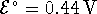 at 298K.
at 298K.
- Calculate the equilibrium constant for this reaction.
[6 marks]
- If we start with 10L of a 2.5mol/L solution of
chromium (III), what mass of solid chromium is produced if
an excess of manganese is tossed into the solution?
Chromium metal has a molar mass of 51.996g/mol.
[4 marks]
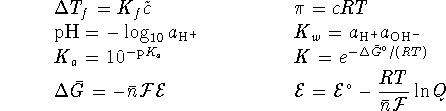
For a first-order reaction,
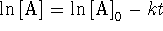 and
and
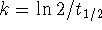 .
For a Brønsted acid,
.
For a Brønsted acid, 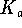 is the equilibrium constant for the reaction
is the equilibrium constant for the reaction
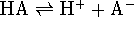 . For a Lewis
acid, it is the equilibrium constant for the reaction
. For a Lewis
acid, it is the equilibrium constant for the reaction 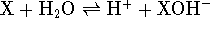 .
.
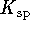 (the solubility product) is the equilibrium constant for
the dissociation of an ionic solid into aqueous ions.
(the solubility product) is the equilibrium constant for
the dissociation of an ionic solid into aqueous ions.
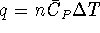 or
or 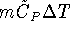
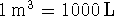
To convert degrees Celcius to Kelvin, add 273.15.
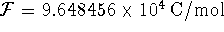
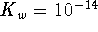 at
at 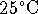

Up: Back to the Chemistry 2000 test
index
Marc Roussel
Tue Jul 8 14:38:19 MDT 1997
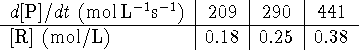
![]()
![]()
![]()
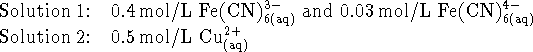
![]()
![]() at 298K.
at 298K.