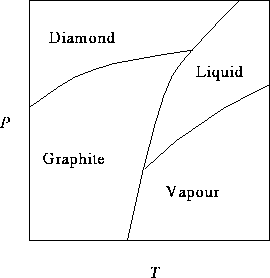
The curve separating the diamond and graphite regions intersects
the P axis at ![]() and the curve
between the graphite and vapour regions intersects the T axis
at
and the curve
between the graphite and vapour regions intersects the T axis
at ![]() .
.
- It is often cheaper to manufacture industrial diamonds (to be used in cutting tools, for instance) than to mine them. Is it easier to make diamonds at higher or lower temperatures? Explain how you get this information from the phase diagram. [4 marks]
- Does the melting temperature of diamond increase or decrease with pressure? Explain how you can tell from the phase diagram. [4 marks]
- What phase change eventually occurs when graphite is heated at 1atm? (Hint: Carefully consider the scale of the P axis.) [2 marks]
- Explain how you can make water boil at room temperature. Hint: Refer to the phase diagram of water, Fig. 11.49 of your textbook. [5 marks]
- Explain the physical principles behind overnight dew formation. [5 marks]