Welcome to the Research Website
of
Peter W. Dibble
Bringing you cooler molecules since
1987.
Contact information:
Department of Chemistry and Biochemistry
University of Lethbridge
Lethbridge
AB Canada, T1K 3M4
Dibble@uleth.ca
Phone: Office: 329-2305; Lab: 329-2308
The Molecular Hall of Phane
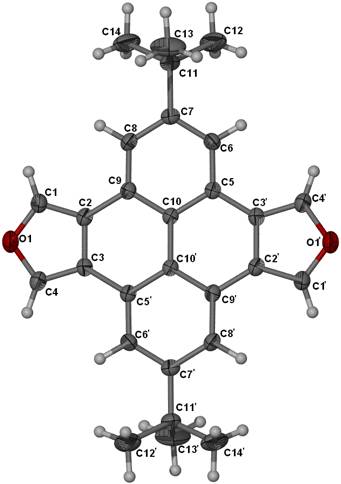
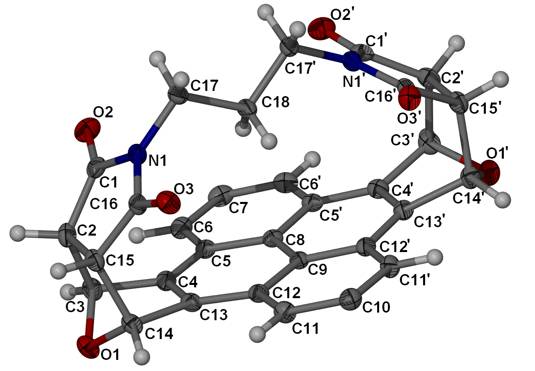
On the left is the X-ray structure of
pyreno[4,5-c;9,10-c’]difuran. This
was used to make the molecule on the right, a pyrenophane. It’s an example of a cyclophane: molecules that have a flat aromatic
portion linked across the ring by an aliphatic chain. These were prepared by David Franz; X-ray structures by
Steve Robbins and René Boeré.
Dr. Dibble’s
Publication List:
25) “Synthesis and
Characterization of 2,7-Di(tert-butyl)pyreno[4,5-c:9,10-c’]difuran and Derived
Pyrenophanes”, David Franz, Steven J. Robbins, René T. Boeré and Peter W.
Dibble*, J. Org. Chem, 2009, in
press.
24) “X-ray
Structures of Cyclophanes Derived from Naphtho[1,2-c:5,6-c]difuran and the
Synthesis, Structure and Reaction Kinetics of its 1,3,6,8-Tetrasilylated
Derivative”, Steven J. Robbins, Michelle E. Thibault, Jason D. Masuda, David R.
Ward, René T. Boeré and Peter W. Dibble, J. Org. Chem., 2009, 74, pp. 5192-5198.
23) “Stable
phenylene- and biphenylenebis(isobenzofuran)s related to
diphenylisobenzofuran”, Sheri A. Klassen, Richard Boehme, Sean D. Derrick,
Klaus Moock, A. Elizabeth Baker, Deryn E. Fogg, René T. Boeré and Peter W. Dibble, Can. J. Chem., 2009, 87, pp 738-744.
22) “4,14:7,11-Diepoxy-2,9-propanonaphtho[1,2-f:5,6-f']diisoindole-1,3,8,10-tetrone,3a,4,7,7a,10a,11,14,14a-octahydro(2R,3aR,4S,7R,7aS,9R,10aR,11S,14R,14aS)-rel-
(9CI), a cyclophane derived from naphtho[1,2-c:5,6-c]difuran”, Michelle E.
Thibault, Masood Parvez and Peter W. Dibble,
Acta Cryst. (2008). E64, o1837.
21) “Furans
and their Benzo Derivatives: Applications”, Peter W. Dibble, Matthew Letts and
Brian A. Keay, “Comprehensive Heterocyclic Chemistry III”, 2008, Elsevier.
20) “1,3-Diphenylisobenzofuran”,
Reneę T. Boereę,* Peter W. Dibble and Kristapher E. Fischer, Acta Cryst. (2008) E64, o686.
19) “Structures
of the 2:1 adducts of benzyne with 2-methylanisole and benzene”, C. O. Bender,
R. T. Boeré, P. W. Dibble, R. T. McKay,
Can. J. Chem., 2007, 85, 461-465.
18) “Diels-Alder
Reactivity of Benzannulated Isobenzofurans as assessed by Density Functional
Theory”, Davor Margetic*, R. N.
Warrener and Peter W. Dibble, Journal of Molecular Modeling, Springer,
13.01.2004, vol. 0, no. 2, pp. 87-93.
17) "Naphtho[l,2-c:5,6-c]difuran:
reactive linker and cyclophane precursor", Taunia L. L. Closson, Sidney
Manning, Michelle E. Thibault, and Peter W. Dibble*, J. Org. Chem. 2003, 68, 8373-8378.
16) “The
synthesis and photochromic properties of molecules containing [e]- annelated
dihydropyrenes. Two and three way pi-switches based on the
dimethyldihydropyrene-metacyclophanediene valence isomerization”; Reginald H.
Mitchell,* Timothy R. Ward, Yongsheng Chen, Yunxia Wang, S. Ananda Weerawarna,
Peter W. Dibble, Michael J Marsella, Adah Almutairi, and Zhi-Qiang Wang; J.
Am. Chem. Soc., 2003, 125,
2974-2988.
15) “Phenanthro[2,3-c]furan
– A Stable Benzologue of Isobenzofuran with Greater Reactivity”, Michelle
Thibault, Laurie A. Pacarynuk, Taunia L. Closson and Peter W. Dibble*, Tetrahedron Lett, 2001, 42, 789-791.
14) “pi-Switches: Synthesis of Three-Way molecular
Switches Based on the Dimethyldihydropyrene-Metacyclophanediene Valence
Isomerization”, Reginald H. Mitchell, Timothy R. Ward, Yunxia Wang and Peter W.
Dibble, J. Am. Chem. Soc. 1999, 121, 2601-2602.
13) “Naphtho[1,2-c:5,6-c]difuran,
a Stable Isobenzofuran Derivative”, Daniel W. Yu, Kathryn E. Preuss, Paulette
R. Cassis, Dontem Dejikhangsar and Peter W. Dibble, Tetrahedron Letters, 1996, 49, 8845-8848.
12) “Furans
and Their Benzo Derivatives:
Applications”, Brian A. Keay and Peter W. Dibble, Comprehensive
Heterocyclic Chemistry II, Volume 2, Chapter 8, pp. 395-436, Pergammon Press, Oxford,
1996.
11) “Steric
and Electronic Effects in Imine/Hemiaminal Ring-Chain Tautomerism”, Sean D.
Derrick, Richard Boehme, Ken M. Wong, Frank Nemeth, Kelly Tanaka, Brian
Rumberg, Richard A. Beekman and Peter W. Dibble, Tetrahedron, 1996, 52, 7679-7690.
10) “The
Generation of Anthra [2,3-c]furan via Aromatic-Ring Homologation of
Naphtho[2,3-c]furan”, Noah P. W. Tu, Judy C. Yip and Peter W. Dibble, Synthesis, 1996, 77-81.
9) “Teraryls
via Phenylenebis(isobenzofuran)s”, Susan Leong-Neumann, Sean D. Derrick and
Peter W. Dibble, Tetrahedron Lett. 1995, 36, 4181,
8) “High-pressure
Intramolecular Diels-Alder Reactions of the Furan Diene”, Brian A. Keay and
Peter W. Dibble, Tetrahedron Lett. 1989, 30, 1045.
7) “The
EI Mass Spectra of Isobenzofurans and Adducts Therefrom”, Peter W. Dibble and
Russell Rodrigo, Org. Mass. Spec. 1988, 30, 1045.
6) “1-Phenylisobenzofuran,
1-Phenylnaphtho[2,3-c]furan, 1-Phenylnaphtho[1,2-c]furans”, James G. Smith,
Deryn E. Fogg, Ian J. Munday, Richard E. Sandborn and Peter W. Dibble, J.
Org. Chem.
1988,
53,
2942-2953.
5) “Polycyclic
aromatic hydrocarbons via 1-(arylmethyl)isobenzo- and -naphtho[2,3-c]furans”,
James G. Smith, and Peter W. Dibble, J. Org. Chem., 2008, 53, 1841-1848.
4) “The preparation and
reactions of naphtho[1,2-c]furan and naphtho[2,3-c]furan”, James G. Smith, Richard E. Sandborn and
Peter W. Dibble, J. Org. Chem. 1986, 51, 3762-3768.
3) “Ring-chain
tautomerism of 1-hydroxyphthalans. An examination of structural effects”, James
G. Smith and Peter W. Dibble, Tetrahedron, 1984, 40, 1667-1672.
2) “Naphtho[1,2-c]furan
and Naphtho[2,3-c]furan”, James G. Smith and Peter W. Dibble, Chem. Comm., 1983, 1197.
1) “2-(Dimethoxymethyl)benzyl
alcohol: a convenient isobenzofuran precursor”, James G. Smith and Peter W.
Dibble, J. Org. Chem., 1983, 48, 5361-5362.